Generic Drug Approval: What Really Happens Before Your Prescription Gets Filled
When you pick up a generic drug approval, the official process the U.S. Food and Drug Administration (FDA) uses to verify that a generic version of a brand-name drug is safe, effective, and identical in performance. Also known as ANDA (Abbreviated New Drug Application), it’s the backbone of affordable medicine in the U.S. This isn’t just paperwork—it’s a strict scientific gatekeeping system that ensures your $5 generic pill does the exact same job as the $50 brand name. The FDA doesn’t just accept claims. They demand proof.
Here’s the key: for a generic drug to get approved, it must match the brand-name version in active ingredient, the chemical compound responsible for the therapeutic effect, dosage form, whether it’s a tablet, capsule, injection, or liquid, and bioequivalence, how quickly and completely the drug enters your bloodstream. That last one matters most. A generic must deliver the same amount of active ingredient into your blood at the same rate as the original—within 80% to 125% of the brand’s levels. No wiggle room. This isn’t theory. It’s tested in real people under controlled conditions. If it doesn’t pass, it doesn’t get approved.
But here’s what most people don’t realize: the FDA allows differences in inactive ingredients, the fillers, dyes, and preservatives that hold the pill together or help it dissolve. That’s why some people report different side effects or allergies with generics. It’s not the drug working differently—it’s the extra stuff. And that’s why your pharmacist might ask if you’ve had reactions to certain dyes or lactose before switching. The FDA doesn’t require these to match, but they do require them to be safe.
Some people still think generics are "second-rate" because of media stories or anecdotal complaints. But the truth? Over 90% of prescriptions filled in the U.S. are generics. And the FDA has reviewed more than 20,000 generic drugs since 1984. Every single one had to prove it works the same. No exceptions. If you’re on a generic for high blood pressure, thyroid medication, or antibiotics, you’re getting the same clinical result as the brand—just without the marketing budget.
What you’ll find below are real stories from patients and experts who’ve seen the gaps between perception and reality. From how media distorts the truth about generics, to why some doctors still hesitate to prescribe them, to how price wars and international pricing affect what ends up on your shelf. You’ll also learn what to watch for if you notice a change after switching, how to report unexpected side effects, and why the FDA’s approval process is more rigorous than most people think. This isn’t about theory. It’s about what’s in your pill bottle—and why you can trust it.
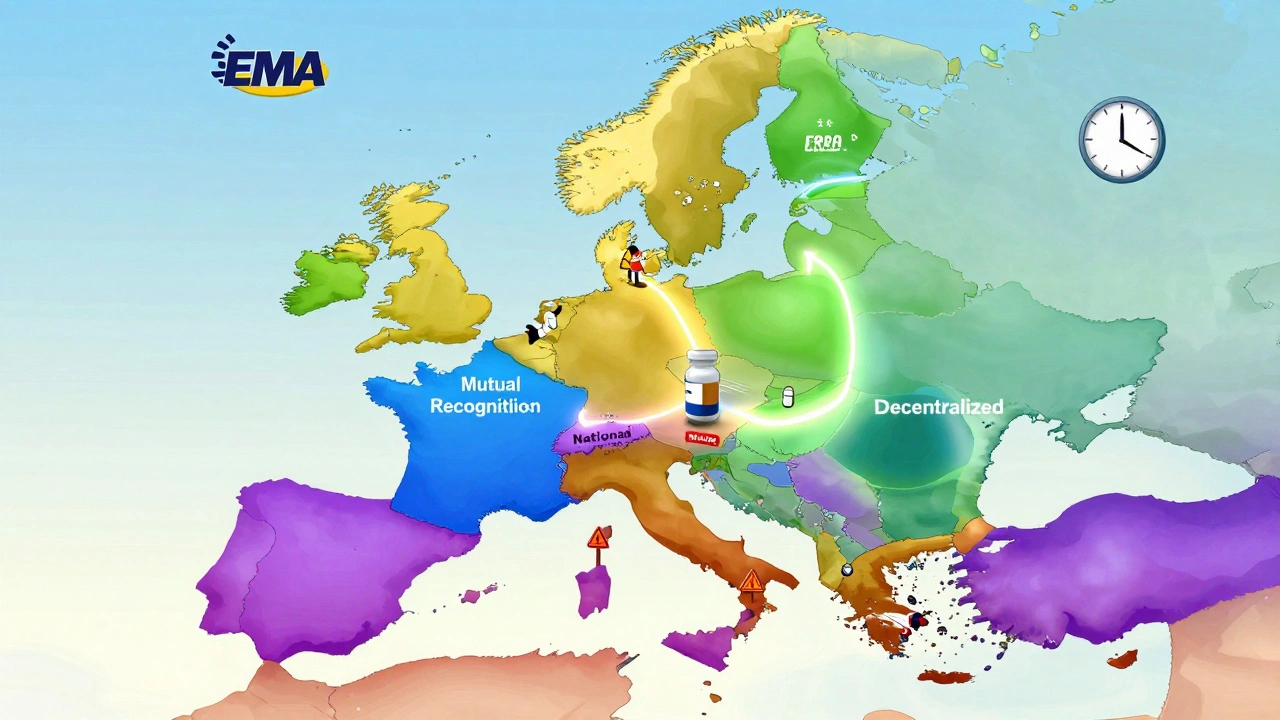
European Generic Markets: Regulatory Approaches Across the EU in 2025
The EU's generic drug market is undergoing major regulatory changes in 2025, with new approval pathways, faster market entry rules, and stricter supply obligations. Learn how the system works-and who wins and loses under the reforms.