1 Dec 2025
- 15 Comments
When a patient in Germany, Spain, or Poland picks up a generic version of a brand-name drug, they’re not just saving money-they’re participating in one of the most complex regulatory systems in the world. The European Union’s approach to generic medicines isn’t a single rulebook. It’s a patchwork of four different approval paths, each with its own timeline, cost, and hidden traps. And as of 2025, everything is changing.
Four Ways to Get a Generic Drug Approved in the EU
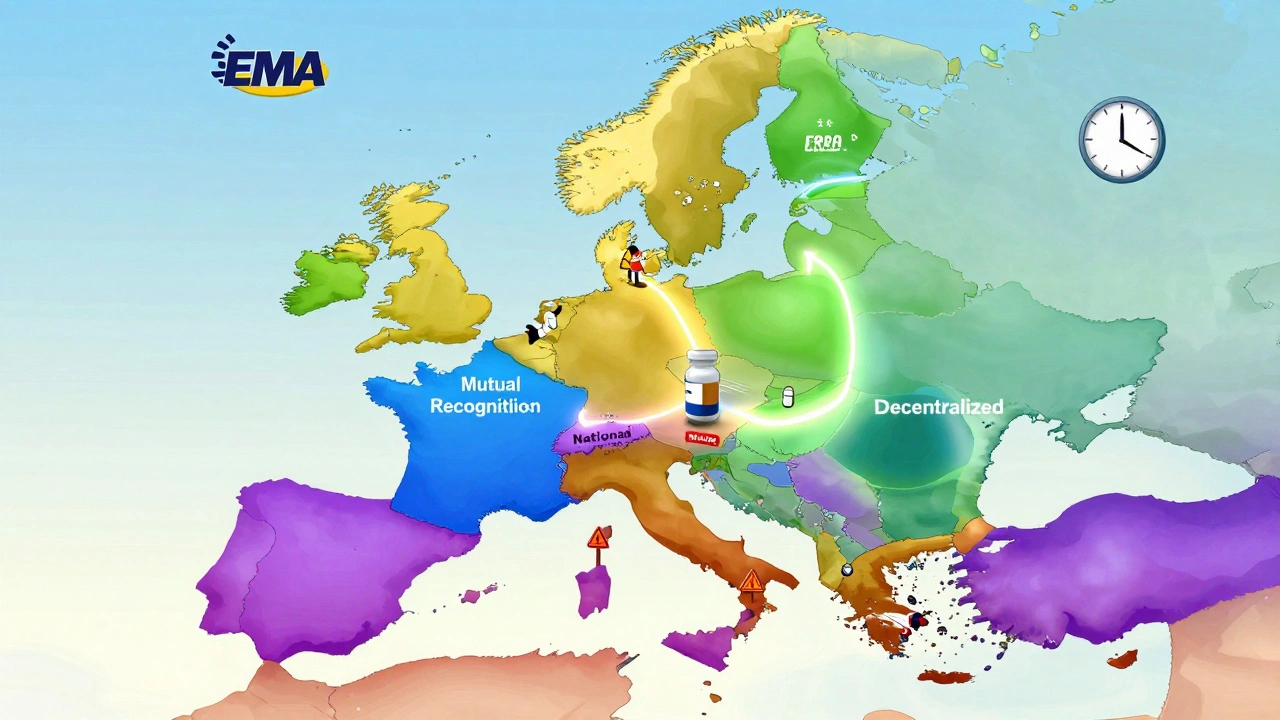
There’s no one-size-fits-all route to selling a generic drug across the EU. Manufacturers must choose from four approval pathways, and the decision can make or break their market entry.
The Centralized Procedure is the fastest way to launch across all 27 EU countries, plus Iceland, Liechtenstein, and Norway. It’s used for about 15% of applications, mostly by big players like Sandoz or Viatris. You file once with the European Medicines Agency (EMA), and if approved, you get a single authorization valid everywhere. The process used to take 210 days for scientific review. Now, under the 2025 Pharma Package reforms, it’s cut to 180 days. The catch? It costs between €1.6 million and €2.2 million total-application fees, consultancy, and studies combined. Only worth it if you’re targeting a high-volume drug with projected sales over €250 million across the EU.
The Mutual Recognition Procedure (MRP) is the middle ground. You get approved in one country-the Reference Member State-then ask others to accept it. It’s used in 42% of cases. Sounds simple, right? Not quite. Even if the EMA says the drug is safe, national regulators can drag their feet. A 2024 IQVIA report found MRP approvals took an average of 132.7 days, not the 90-day target. Teva’s experience with generic rosuvastatin showed how one country’s pricing delay can hold up launches in three others by over eight months.
The Decentralized Procedure (DCP) lets you apply to multiple countries at once, without prior approval anywhere. It’s used in 38% of cases. But coordination is a nightmare. Each country can raise objections, and when they do, the 210-day clock resets. A 2024 GMDP Academy study found 37% of DCP applications faced delays longer than six months, especially in Eastern Europe, where quality standards for things like tablet coatings or impurity levels aren’t always aligned with EMA guidelines.
The National Procedure is the slowest and most limited. You apply only to one country. It’s used in just 5% of cases-usually when a company wants to test the waters in a high-reimbursement market like France or Italy. It takes 180 to 240 days, and you get no access to other countries. Accord Healthcare found that applying nationally in France took 197 days, while using MRP for the same drug across five countries took just 142 days. Why bother? Sometimes, you’re targeting a niche or trying to avoid the bureaucracy of cross-border approval.
What Makes a Generic Drug Approved?
No matter which path you take, the EMA’s requirements are strict. To be approved, a generic must be identical to the original brand drug in three key ways:
- Same active ingredient, in the same amount
- Same pharmaceutical form-tablet, injection, inhaler, etc.
- Proven bioequivalence
Bioequivalence means your generic must deliver the same amount of medicine into the bloodstream at the same rate as the brand. The EMA requires clinical studies showing that the 90% confidence interval for two key metrics-Cmax (peak concentration) and AUC (total exposure)-must fall between 80% and 125% of the original drug. This isn’t a suggestion. It’s a hard rule. And in 2025, the EMA updated its bioequivalence guidelines to be even more precise, especially for complex generics like inhalers or extended-release tablets.
That’s where things get messy. Germany’s BfArM, for example, now requires additional pharmacodynamic studies for inhalers beyond what the EMA demands. France’s ANSM wants extra documentation on pediatric formulations. If your generic is made from a polymorphic compound (a crystal form that can change stability), Germany requires extra stability testing. These national add-ons aren’t listed in the EMA’s main rules. You have to know them-or pay someone who does.
The 2025 Pharma Package: What’s Changing?
The biggest shift in EU generic regulation in 20 years came with the Pharma Package reforms, finalized on June 4, 2025. Three changes are already reshaping how generics enter the market.
First, the Bolar exemption has been expanded. Before, generic companies could start negotiating prices and reimbursement with health systems only two months before a patent expired. Now, they can start six months earlier. That’s a game-changer. REMAP Consulting’s 2025 model shows this alone can cut launch delays by 4.3 months on average. It also gives payers more leverage-hospitals and insurers can start comparing prices before the generic even hits shelves. The result? Launch prices could drop 12-18% due to earlier competition.
Second, regulatory data protection is being shortened. Previously, brand-name drugs got 10 years of protection from generic competition (8 years of data exclusivity + 2 years of market exclusivity). Now, it’s 8 years + 1 year. That’s it. You can extend it to 10 years if your drug meets public health targets, like treating rare diseases or improving pediatric use. But for most common generics, the clock starts ticking sooner. Evaluate Pharma estimates this will speed up entry for 78 high-value biologics currently in development.
Third, the obligation to supply rule is now binding. If a generic manufacturer is the only source of a medicine and starts cutting supply, national authorities can step in. But here’s the problem: every country defines “sufficient quantities” differently. Professor Panos Kanavos of LSE Health warns this could create artificial shortages in smaller markets, where companies decide it’s not worth the effort to supply low-volume countries.
Who’s Winning in the EU Generic Market?
The EU generic market was worth €42.7 billion in 2024, growing at 6.2%-and it’s getting more competitive. Indian manufacturers now account for 38% of all EU generic approvals, up from 29% in 2020. Companies like Dr. Reddy’s, Sun Pharma, and Cipla are moving fast, using the DCP and MRP to target multiple countries at once with low-cost production.
European firms like Sandoz and Viatris are fighting back-not by being cheaper, but by being smarter. They’re using the Centralized Procedure for blockbuster generics. Sandoz’s launch of the generic version of Novartis’s Cosentyx in Q2 2025 was simultaneous across all EU markets-11 months faster than MRP would have allowed. That kind of speed matters when you’re trying to capture market share before price cuts begin.
But the real winners? The payers. Health systems across the EU are now better positioned to negotiate. With the Bolar exemption, they can start comparing prices six months before launch. With the obligation to supply, they can threaten to revoke licenses if companies cut back. And with the new Joint HTA Regulation (effective January 1, 2025), they can now share health technology assessments across borders. One country’s analysis of cost-effectiveness can be used by others, reducing duplication and speeding up reimbursement.

What Generic Companies Are Struggling With
A 2025 survey by the Association of the British Pharmaceutical Industry found that 68% of generic manufacturers see inconsistent national requirements as their biggest headache. It’s not the EMA rules they can’t meet-it’s the extra stuff countries add on top.
My company, Viatris, spent €3.2 million extra in carrying costs on one MRP launch because of delays in Germany and Belgium. Accord Healthcare’s Maria Papadopoulou said at the 2025 EGA conference that every time a country objects to a DCP application, the 180-day clock restarts. That means a product that should launch in 6 months could be stuck for over a year.
Then there’s the new ePI requirement. By 2026, all product information must be submitted in XML format. That’s not just a paperwork change. It’s a tech overhaul. Companies need new systems, new training, and new IT teams. White & Case estimates this will cost €180,000-250,000 per company. Smaller firms can’t afford it. That’s why some analysts predict the reforms will benefit big players and hurt mid-sized generics.
What’s Next for Generic Drugs in Europe?
The 2025 reforms are just the beginning. The Critical Medicines Act, passed in March 2025, now requires member states to stockpile 200 essential generic medicines. That sounds good-until you realize the quality checks for those stockpiles are stricter than ever. It’s another layer of compliance.
Meanwhile, the US-EU Framework Agreement, effective September 1, 2025, could change the cost of raw materials. Tariffs on active pharmaceutical ingredients might rise or fall, depending on the compound. No one knows yet how it will impact pricing, but manufacturers are already adjusting their supply chains.
By 2028, generic prescriptions in the EU are projected to jump from 65% to 69.2% of all prescriptions by volume. That’s good for patients. But for manufacturers? The game is harder than ever. You need to know not just the EMA rules, but also the hidden expectations of 27 national regulators. You need to pick the right approval path, manage multi-country timelines, and invest in tech just to file paperwork. And you have to do it all faster, cheaper, and with more transparency than before.
The EU didn’t make its generic system easy. It made it efficient-for those who know how to play it.
How long does it take to get a generic drug approved in the EU?
Approval times vary by pathway. The Centralized Procedure takes 180 days under 2025 rules. The Mutual Recognition Procedure averages 133 days but can stretch longer due to national delays. The Decentralized Procedure takes about 247 days on average, often longer if countries raise objections. The National Procedure takes 180-240 days. The 2025 Bolar exemption now lets companies start pricing talks six months before patent expiry, which can shorten actual market entry by up to 4.3 months.
What’s the difference between the Centralized and Mutual Recognition Procedures?
The Centralized Procedure gives you a single approval valid across the entire EU in one go, managed by the EMA. It’s faster for multi-country launches but costs over €1.6 million. The Mutual Recognition Procedure starts with approval in one country, then asks others to accept it. It’s cheaper-around €180,000-220,000-but slower and riskier, because any country can delay or reject your application even after the EMA approves it.
Why do some countries delay generic approvals even after EMA approval?
National regulators can impose additional requirements not found in EMA guidelines. For example, Germany requires extra pharmacodynamic studies for inhalers. France demands pediatric formulation details. Poland and Romania sometimes request different impurity profiles. These are legal, but they’re not standardized. The EU doesn’t force them to align, so delays happen even when the drug meets all EU-wide standards.
Are Indian generic manufacturers dominating the EU market?
Yes. Indian companies accounted for 38% of all EU generic approvals in 2024, up from 29% in 2020. They’re winning by using lower-cost manufacturing and targeting multi-country pathways like MRP and DCP. But European firms like Sandoz and Viatris still hold 52% of the market share by using the Centralized Procedure for high-value drugs, where speed and simultaneous launch matter more than cost.
What’s the impact of the 2025 Bolar exemption on generic drug prices?
The extended Bolar exemption lets generic makers start pricing negotiations six months before patent expiry. This gives health systems more time to compare prices and negotiate discounts before the drug launches. As a result, launch prices are expected to drop 12-18% due to earlier competitive pressure. It also helps payers avoid last-minute price spikes and gives them leverage to secure better deals.
Do I need to invest in new software to sell generics in the EU after 2025?
Yes. Starting in 2026, all product information (ePI) must be submitted in XML format. This requires new IT systems, data mapping tools, and staff training. White & Case estimates the cost per company at €180,000-250,000. Smaller manufacturers may struggle to afford this, which could push market share toward larger companies with existing digital infrastructure.

ATUL BHARDWAJ
December 1, 2025Indian generics are the real MVPs here. Low cost, high volume, and we know how to navigate MRP/DCP like chess masters. EU regulators keep adding layers, but we adapt faster than their bureaucracy.
Jack Dao
December 2, 2025Wow. So the EU just turned pharmaceutical regulation into a Kafkaesque nightmare for everyone except Big Pharma and Indian conglomerates. Classic. 🤦♂️
Ella van Rij
December 2, 2025Ohhh so THAT’S why my generic blood pressure med tastes like chalk now? Must be the ‘polymorphic compound stability testing’ I missed. 😘
Steve World Shopping
December 4, 2025Regulatory fragmentation = systemic inefficiency. The EU’s non-harmonized national add-ons create transaction cost externalities that distort market entry dynamics. Path dependency is alive and well.
Rebecca M.
December 5, 2025So let me get this straight - I can’t get my generic asthma inhaler approved because Germany wants extra studies… but they still sell me the brand-name version at 10x the price? I’m not mad. I’m just disappointed. 😭
Lynn Steiner
December 5, 2025They’re gonna make us all sick just to save a few bucks. This isn’t healthcare. It’s corporate chess with our lives as pawns. I hate this. 💔
Alicia Marks
December 6, 2025You got this. The system’s broken, but people are still getting life-saving meds. Keep pushing.
Jay Everett
December 6, 2025Let’s be real - the EU didn’t make this system to be fair. It made it to be *strategic*. The Bolar exemption? That’s not generosity - it’s leverage. Paying systems now have six months to lowball prices before the generic even hits the shelf. And guess who’s left holding the bag? The little guys. The ones who can’t afford $250k in IT upgrades just to submit XML. This isn’t reform. It’s consolidation disguised as progress. 🧠
Shannara Jenkins
December 8, 2025Big pharma’s playing the long game, but Indian companies are playing the game the EU forgot existed - speed + scale. Kudos to them. The patients win. 🙌
Elizabeth Grace
December 9, 2025I just spent 4 hours on the phone with my pharmacy because my generic pill looked different. Now I’m crying. Why is this so hard? 😭
Steve Enck
December 10, 2025The 2025 Pharma Package is a textbook case of regulatory capture masked as consumer protection. The expanded Bolar exemption doesn't accelerate access - it enables predatory pricing by institutional payers. The obligation to supply clause is a regulatory trapdoor for state intervention. And the XML mandate? A deliberate barrier to entry designed to favor vertically integrated conglomerates. This is not innovation. It is enclosure.
मनोज कुमार
December 11, 2025Why so many rules? Just let us make the pills. People need them. 🤷♂️
Joel Deang
December 12, 2025so like… xml? like… html but for pills? 😅 i think i need a nap
dave nevogt
December 14, 2025There’s a quiet tragedy here - the EU’s regulatory complexity wasn’t born of malice, but of fear. Fear of unsafe drugs, fear of corporate dominance, fear of national sovereignty clashing with supranational authority. But in trying to balance every variable, they’ve created a system where only those with armies of lawyers and compliance officers can survive. The patient, who just needs the pill, becomes an afterthought in the spreadsheet. The real innovation isn’t in the drug - it’s in the ability to navigate the labyrinth. And that’s not medicine. That’s survival.
Paul Keller
December 15, 2025Let’s not romanticize Indian manufacturers. Yes, they’re efficient. But their cost advantage comes from labor exploitation, environmental laxity, and regulatory arbitrage. The EU’s fragmented system may be messy, but it’s a defense against a race to the bottom. If we want safe, high-quality generics - and we do - then we must accept the cost of oversight. The alternative is a global pharmaceutical Wild West. I’d rather pay more and sleep at night.