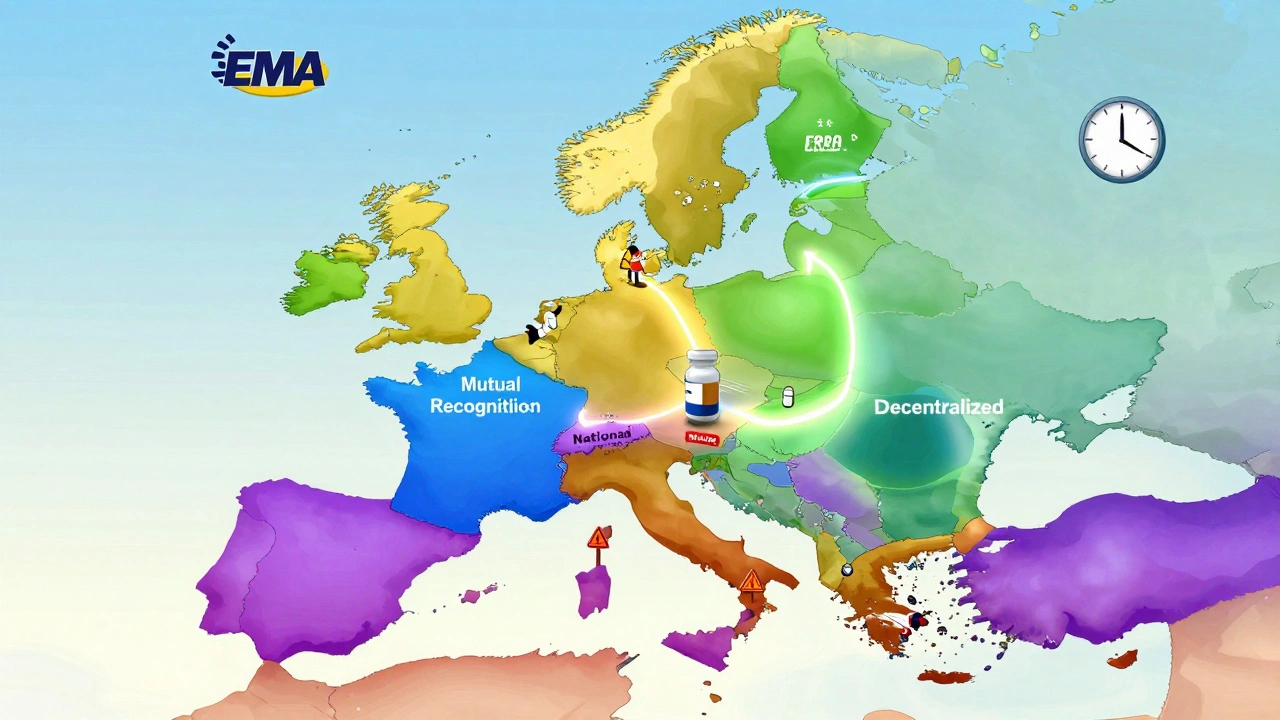
EU Pharmaceutical Regulations: What You Need to Know About Drug Safety and Pricing
When you pick up a pill in Germany, France, or Spain, it didn’t just appear on the shelf—it passed through a strict system called EU pharmaceutical regulations, a unified set of rules governing how medicines are tested, approved, marketed, and monitored across all European Union member states. Also known as European Medicines Agency (EMA) framework, this system ensures that every drug sold in the EU meets high safety, quality, and effectiveness standards before it reaches patients. Unlike the U.S. FDA, the EU doesn’t just approve drugs country by country—it evaluates them once for all 27 member states, cutting red tape and making sure everyone gets the same level of protection.
These rules don’t just cover new drugs. They also control how generic drug pricing, the process by which countries set lower prices for off-patent medications by comparing costs across borders. Also known as international reference pricing, it’s why your generic statin might cost €2 in Portugal and €5 in Sweden—the system forces manufacturers to compete on price, but sometimes leads to shortages when profits vanish. And it’s not just about cost. medication approval, the multi-year process of clinical trials, data review, and post-market surveillance required before a drug can be sold. Also known as marketing authorization, it’s where the EMA steps in to review everything from rare side effects to how well a drug works in older adults or people with kidney disease. That’s why you see warnings on labels for things like adrenal insufficiency from opioids or reduced levothyroxine absorption from soy—those risks were flagged during approval or later through EU-wide reporting systems.
Behind every boxed warning, every generic pill, every price drop you’ve seen, there’s a chain of EU regulations making sure it’s legal, safe, and traceable. You might not see the paperwork, but you feel the results: fewer dangerous interactions, clearer labels, and more consistent access to medicines whether you live in Lisbon or Warsaw. These rules also force transparency—pharmacies must report adverse events, manufacturers must update safety data, and patients can track where their meds came from. The system isn’t perfect—shortages happen, prices still vary, and some doctors still distrust generics—but it’s the reason you can trust your prescription more than ever before.
Below, you’ll find real-world examples of how these regulations touch your life—from how a drug gets approved to why your generic pill might have different fillers, how price controls affect availability, and what to do when a medication doesn’t work as expected. This isn’t theory. It’s what’s on your bottle, in your doctor’s notes, and in the pharmacy’s back room.
European Generic Markets: Regulatory Approaches Across the EU in 2025
The EU's generic drug market is undergoing major regulatory changes in 2025, with new approval pathways, faster market entry rules, and stricter supply obligations. Learn how the system works-and who wins and loses under the reforms.