Generic Market Access: How Drugs Reach Patients and Why Prices Drop
When we talk about generic market access, the process by which affordable versions of brand-name drugs become available to patients through pharmacies, insurers, and public health systems. Also known as generic drug availability, it’s what lets someone pay $4 for a month’s supply of metformin instead of $300. This isn’t just about cost—it’s about whether people can actually get the medicine they need when they need it.
Behind every low-priced generic is a complex system. international reference pricing, a method where countries set drug prices by comparing what other nations pay. Also known as price benchmarking, it’s used by Canada, the UK, and Australia to keep costs down—but it sometimes leads to shortages when manufacturers decide the profit isn’t worth the effort. Then there’s pharmaceutical pricing, how companies and governments decide what a drug should cost after patent expiry. Also known as post-patent pricing, it’s where price wars happen: one generic maker drops their price to $0.10 per pill, and suddenly everyone else has to follow—or lose the contract. These aren’t abstract policies. They directly affect whether your local pharmacy has your blood pressure pill in stock.
And it’s not just about money. generic medications, chemically identical to brand-name drugs but often sold without marketing hype. Also known as non-branded drugs, they’re trusted by doctors and regulators—but public doubt lingers because of bad headlines or rare side effects from inactive ingredients. That’s why media coverage and patient education matter just as much as pricing. A person might refuse a generic not because it doesn’t work, but because they’ve heard a story about someone who had a reaction. That’s a trust problem, not a science problem.
What you’ll find below are real stories from people who’ve dealt with these issues: why their prescription suddenly cost more, how a generic ran out at their pharmacy, why their doctor still hesitates to prescribe one, and how countries are trying to fix broken systems. These aren’t theory pieces. They’re about what actually happens when a pill goes from factory shelf to your medicine cabinet—and who wins or loses along the way.
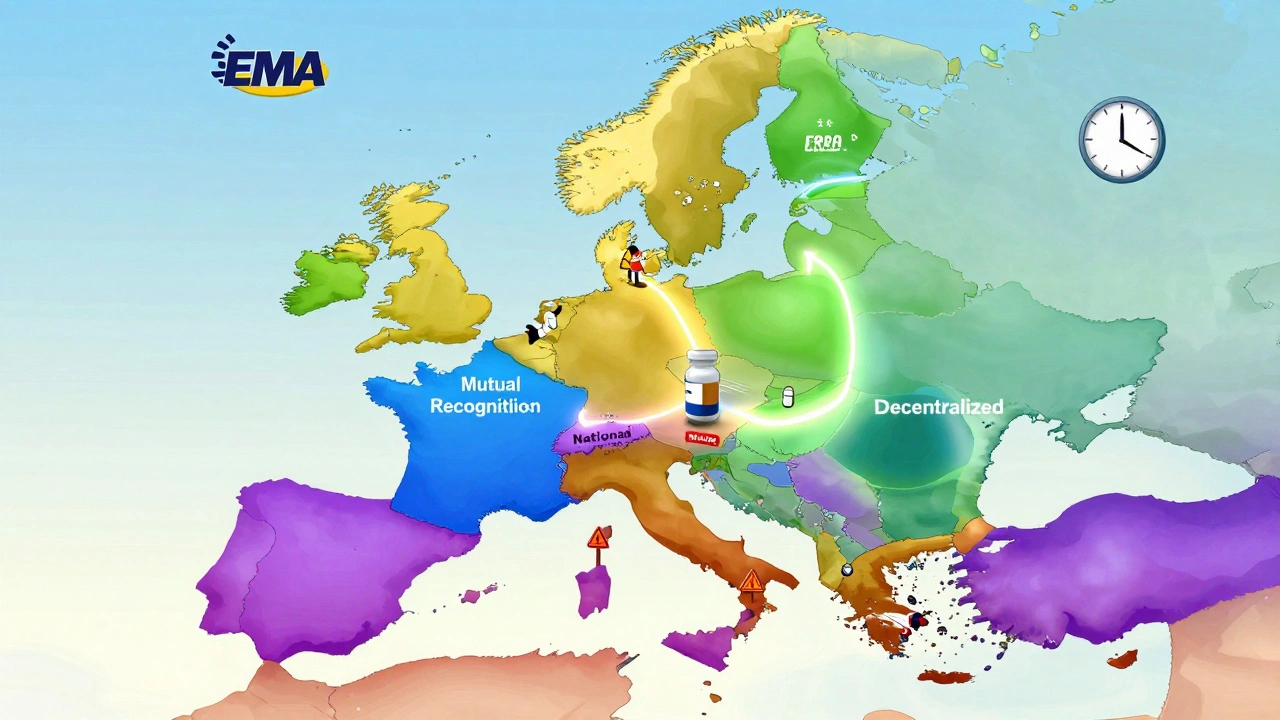
European Generic Markets: Regulatory Approaches Across the EU in 2025
The EU's generic drug market is undergoing major regulatory changes in 2025, with new approval pathways, faster market entry rules, and stricter supply obligations. Learn how the system works-and who wins and loses under the reforms.